Deciphering the codes of drug discovery: application of machine learning in designing compound libraries – practical and legal comments
Publication date: October 14, 2024
On October 10, 2024, Karol Wolak, of KG LEGAL Kiełtyka Gładkowski took part in the event “Deciphering the codes of drug discovery: application of machine learning in designing compound libraries” organized by Selvita as part of the cyclical meetings of LifeScience Kraków Cluster.



The course of the meeting
The speakers: Szymon Czaplak and Fabrizio Ambrogi presented the operation of the TADAM (Target Aware) service Drug Activity Model, i.e. artificial intelligence, which helps find the right drug faster.
TADAM is used in the earliest phases of drug discovery. To begin work on finding the right therapeutic agent, you first need to find an element that exhibits disease-related activity. The TADAM model uses machine learning to leverage artificial intelligence in a high-throughput screening process, i.e. selecting the right library of molecules. The program learns the interactions between proteins and other molecules through exposure to data (machine learning), which allows it to narrow down a large database of compounds into a smaller, potentially active set of compounds. The deep learning model used is able to predict the activity of a compound for a given target, which allows screening of a very large library of compounds and understanding which parts of those compounds are most important for a given case.
The authors presented the research using an example[1].
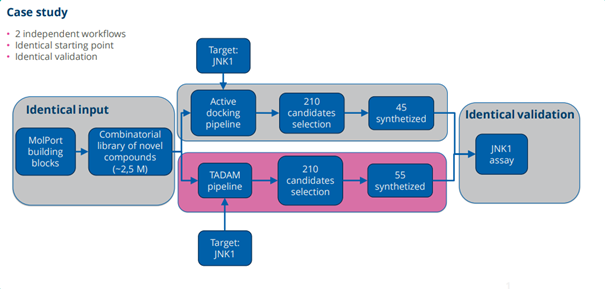
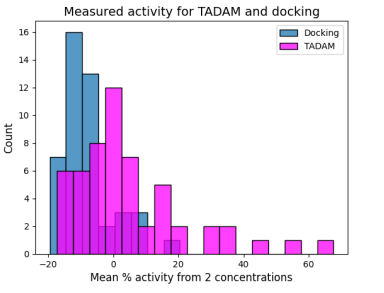
First, a library of chemical compounds (2.5 million new molecules) was taken. Then, using TADAM and Active docking models (without AI), there was checked which molecules fit a specific target – in this phase, there was undertaken, according to the speakers, the analysis how well the molecules can act on the disease. In both cases, 210 of the best molecules were selected, which have the greatest potential. The selected molecules were synthesized in the laboratory – 45 were synthesized from the study derived from the Active docking method, and 55 from the study using the TADAM program. The results of the studies confirmed the authors’ thesis – molecules from the TADAM computer model had much better efficacy. Only one of the molecules derived from Active docking was in the top ten of all the molecules synthesized, and their average activity was much weaker.
The Importance of AI in Drug Discovery
Nowadays, artificial intelligence is increasingly used in research on the search for new drugs. There is available information about the number of molecules developed with the help of AI. Boston Consulting Group prepared a report on this issue[2]. The authors of the review confirmed that thanks to AI, we can expect a doubling of the overall productivity of pharmaceutical research and development. Artificial intelligence can help reduce the costs of research and increase the number of new drugs introduced to the market. However, it should be remembered that the molecules discovered by AI are still in the testing phase, but the results are satisfactory so far.
Legal Considerations on AI-Discovered Medicines in the Context of EU Law
The complex problem of artificial intelligence in the discovery of new drugs should be considered from a legal perspective. Artificial intelligence issues are not currently directly regulated in Polish law. In this case, the provisions of Regulation (EU) 2024/1689 of the European Parliament and of the Council of 13 June 2024 laying down harmonised rules on artificial intelligence and amending Regulations (EC) No 300/2008, (EU) No 167/2013, (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1139 and (EU) 2019/2144 and Directives 2014/90/EU, (EU) 2016/797 and (EU) 2020/1828) commonly known as the AI Act should be taken into account. According to Article 113 of the AI Act, the provisions of this regulation shall apply from 2 August 2026 with certain exceptions. This means that the vacatio legis is slowly coming to an end. Member States should prepare their legal systems for the new provisions, and companies should start risk analysis. In particular, Article 5 of the AI Act, which deals with prohibited practices in the field of AI, should be taken into account. Companies discovering drugs must also check whether their product will be classified as a high-risk system, which is dealt with in Article 6 of the AI Act. Paragraph 2 of this article refers to Annex III, which explicitly lists high-risk AI systems. Paragraph 1 also defines what such a system is, but in a different way – the provision specifies two conditions that must be met cumulatively.
Chapter III, Section 2 of the Regulation specifies requirements for high-risk AI systems – if the program were to be classified as such, pharmaceutical companies would have to be aware of more obligations imposed on them. Article 9, Section 1 of the AI Act informs about the risk management system that must be established, implemented, documented and operated. Section 2 defines this system: a risk management system means a continuous, iterative process, planned and implemented throughout the life cycle of a high-risk AI system, requiring regular systematic review and updating.
Companies offering their contractors access to the AI program are, in accordance with the provisions of the regulation, “suppliers“. The definition is included in Article 3, point 3) of the AI Act – a natural or legal person, public authority, agency or other entity that develops an AI system or a general purpose AI model or commissions the development of an AI system or a general purpose AI model and who – for a fee or free of charge – under their own name or trademark, places on the market or puts into service the AI system. The regulation imposes further requirements on suppliers, but differentiates suppliers of high-risk AI systems (Article 16 of the regulation) from suppliers of general purpose AI models (Article 53 of the regulation). If pharmaceutical companies decide to make their AI system available, they must be aware of the existence of these requirements.
Medicines created by AI should be analyzed in the context of Regulation (EU) No 536/2014 of the European Parliament and of the Council of 16 April 2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EC (hereinafter referred to as the “Regulation”). The Regulation regulates the process of creating new medicines – the phase of research on a new substance. According to Article 4 of the Regulation, a clinical trial is subject to scientific and ethical evaluation and requires authorization in accordance with this Regulation. To start the research, an application must be submitted to the appropriate body by the sponsor. According to Article 2, point 14) of the Regulation, the sponsor is a natural person, company, institution or organization that is responsible for initiating a clinical trial, managing it and organizing its financing. In Poland, applications are submitted to the President of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products and the relevant bioethics committee. The provisions of the Regulation give Member States a large degree of independence in terms of the functioning of the committee. In accordance with Article 2(14) of the Regulation, “ethics committee” means an independent body established in a Member State in accordance with the law of that Member State and authorised to issue opinions for the purposes of this Regulation, taking into account the views of laypersons, in particular patients or patient organisations .
The use of AI in drug discovery may arouse emotions in the context of ethics. The aim of the directive, according to recital 4 of the regulation, is to simplify and harmonize the administrative rules governing clinical trials in the Union, which is associated with accelerating the entire process of creating new drugs. Thanks to AI, it is possible to select appropriate active compounds more quickly, causing the transition to the next phase of testing new substances. This is beneficial from the point of view of science and patients waiting for new therapies.
In addition, it has been shown that the search for the right molecule is more efficient using AI. This means that fewer incorrect molecules will be forwarded to the appropriate clinical trials. From an ethical point of view, this is extremely important, as it will reduce the number of tests that are potentially dangerous to health or unnecessary. This will save health complications due to possible incorrect action of the substance.
The transparency of the algorithm may be a problem. Companies may not want to share the operation of their algorithm, treating it as important information from an economic point of view. In this case, interested parties may not learn the specific reasons for choosing one molecule over another, which also raises ethical questions.
AI-assisted research can bring many benefits, but it is important to remember the ethical challenges that this technology introduces. The ethics committees provided for by the regulation will have to face new problems.
It is worth referring to the concept of good clinical practice, which has been defined in Article 2, point 30 of the Regulation. “Good clinical practice” means a set of detailed requirements regarding the ethics and quality of scientific research, relating to the planning, conduct, execution, monitoring, auditing, recording, analysis and reporting of clinical trials, ensuring the protection of the rights, safety and well-being of participants, and the reliability and robustness of data obtained in clinical trials. According to Article 47 of the Regulation: the sponsor of the clinical trial and the investigator ensure that the clinical trial is conducted in accordance with the protocol and principles of good clinical practice. The method of conducting the research must meet the expectations of those in need and be conducted in a diligent manner, consistent with current scientific knowledge. AI programs must operate without any objections in order to be able to use them in the process preceding clinical trials and during their conduct.
Summary
The event “Deciphering the Codes of Drug Discovery: Applying Machine Learning to Design Compound Libraries” showed that using AI may allow for faster discovery of suitable drugs in the future. Using AI for such a purpose also raises legal issues, which are not clear-cut. The AI Act will soon enter into force, for which Member States and companies offering AI programs must prepare. It will also be necessary to consider issues related to the admission of drugs discovered by AI to clinical trials from not only scientific but also ethical perspectives.
Sources:
https://selvita.com/drug-discovery/integrated-drug-discovery/ai-driven-drug-discovery/
https://lifescience.pl/wydarzenie-klastra/rozszyfrowanie-kodu-odkryania-lekow/
https://toolbox.eupati.eu/resources/odkryanie-i-opracowanie-lekow/?lang=pl
https://www.sciencedirect.com/science/article/pii/S135964462400134X?via%3Dihub#s0040
https://eur-lex.europa.eu/legal-content/PL/TXT/?uri=CELEX:32024R1689
[1]Szymon Czaplak, Tomasz Frączek, Anna Karawajczyk, TADAM: Target Aware Drug Activity Model for focused library design, https://selvita.com/wp-content/uploads/2023/10/TADAM-Target-Aware-Drug-Activity-Model-for-focused-library-design.pdf
[2]Madura KP Jayatunga, Margaret Ayers, Lotte Bruens, Dhruv Jayanth, Christoph Meier, How successful are AI-discovered drugs in clinical trials? A first analysis and emerging lessons, https://www.sciencedirect.com/science/article/pii/S135964462400134X?via%3Dihub#s0040
